| gelly Documentation | previous next |
| Chiral Restraints in gelly |
Copyright © 2007-2016 Global Phasing Ltd.
All rights reserved.
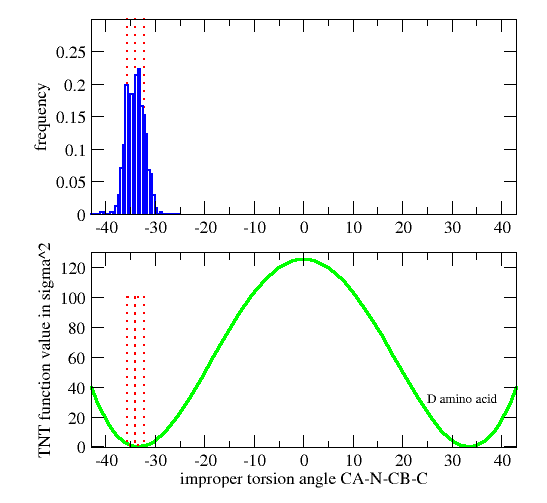
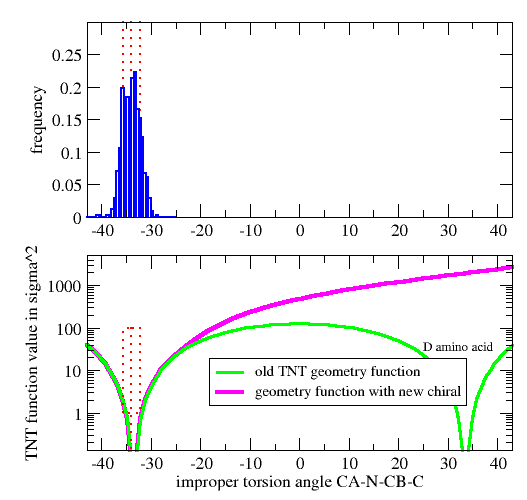
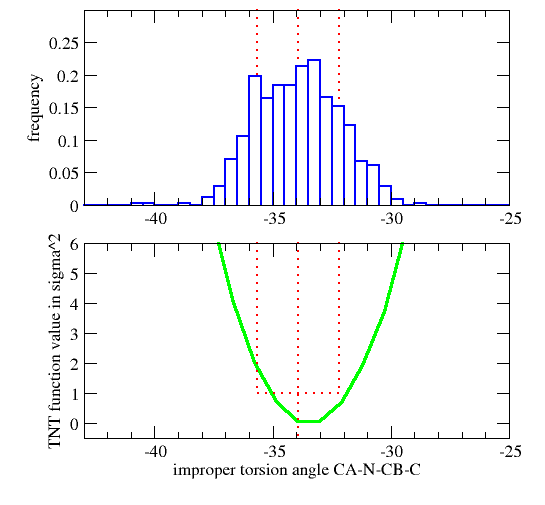
| GEOMETRY ALA CHIRAL 1 1 CA N CB C |
Vchiral = 0.0 if Ωmodel < Ωcutin (Wchiral/σchiral2)*( Ωmodel-Ωcutin)2 otherwise
| WEIGHT CHIRAL 0.0 |
| GEOMETRY DAL CHIRAL 1 1 CA CB N C ! D-amino acid Calpha |