autoBUSTER Example Application: CYS-CYS alternate conformations where one disulfide is bonded and the other broken
Background
- We will use as an example 1osg.
- The protein and lead up autobuster runs to this tutorial are described in the basic NCS setup tutorial.
- After the AutoBusterExample1osgBasicNCS/1osgBasicNCS_003_autoncs_followon (file not found) refinement the strongest difference map features are six strong negative peaks (from -8.2 to -5.6 sigma) centered on each of the disulfide bonds in BAFF protein:
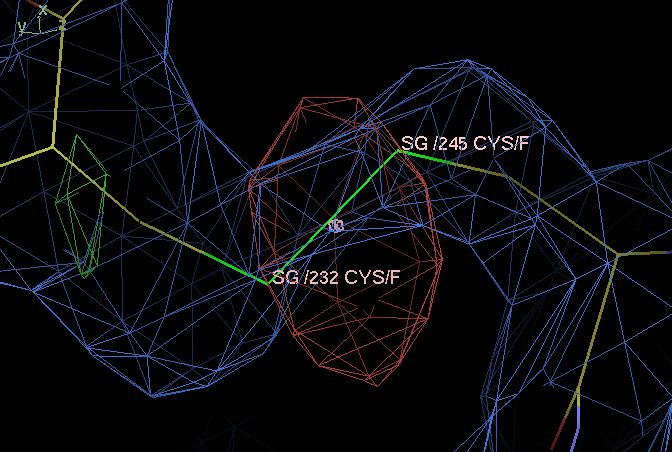
- The situation can arise because of radiation damage resulting a partial reduction of the disulfide.
- How can this be represented in an autoBUSTER refinement? There are two useful approaches:
Starting Files
SSBOND 1 CYS A 232 CYS A 245
SSBOND 2 CYS B 232 CYS B 245
SSBOND 3 CYS C 232 CYS C 245
SSBOND 4 CYS D 232 CYS D 245
SSBOND 5 CYS E 232 CYS E 245
SSBOND 6 CYS F 232 CYS F 245
Possibility A: remove all restraints across the disulfide
- Because the input pdb file does not have SSBOND records for the disulfide bond no bond or angle restraints will be used for the link.
- But because of the lack of the bond the two sulfur atoms across each disulfide would be regarded as having a bad non-bonded contact.
EXCLUDE A|232 A|245
EXCLUDE B|232 B|245
EXCLUDE C|232 C|245
EXCLUDE D|232 D|245
EXCLUDE E|232 E|245
EXCLUDE F|232 F|245
- can be used to turn off the bad contact term across each disulfide. If this is done then no restraints are used between the CYS residues - neither bonded nor nonbonded contact. Essentially the disulfide bond is allowed to lengthen without any penalty.
- results: refinement stats
- So relaxing the bond restraints on the disulfides results in 0.2% drop in both Rwork and Rfree.
- results on structure/maps
Possibility B: create alternate conformations and use utility distance restraints to make one disulfide bonded and the other reduced
- A limitation with the current release is that two alternate structures have to have the same bonding structure.
- page under construction. In the mean time document 1osg_disulfide_break.pdf provides a preliminary description of what can be done.
Page by Oliver Smart original version 29 May 2008. Address problems, corrections and clarifications buster-develop@globalphasing.com
