Pipedream Tutorial 1
(revision 2.0, July 2014)
This tutorial represents a typical and straightforward case, involving two beta-secretase structures, that is run with default options. The reference structure is PDB id: 1W50 and the experimental data is taken from PDB id 4J0P.
| 4j0p.mtz | the structure factors as supplied by the pdb for 4j0p, converted to mtz format |
| 1w50.mtz | the structure factors as supplied by the pdb for 1w50, converted to mtz format |
| 1w50.pdb | the model coordinates as supplied by the pdb for 1w50 |
(A) Restraint dictionary generation
Prior to running Pipedream, we need to generate restraint dictionaries for the ligand(s) soaked into the experimental data and any prosthetic groups present in the structure in addition to the soaked ligand.
Pipedream WILL NOT generate dictionaries itself. Restraint dictionaries should be generated manually and validated before use in an automated procedure.
The experimental structure, 4j0p, was complexed with the compound N-[(4S)-2-amino-4-methyl-5,6-dihydro-4H-1,3-oxazin-4-yl]-4-fluorophenyl-5-cyanopyridine-2-carboxamide (PDB ligand id: 1H8) by soaking.
So, lets generate a restraint dictionary for the ligand, using Grade.
Note that where the ligand is already present and classified by the PDB (as in this case), then a dictionary can be generated using grade_PDB_ligand:
grade_PDB_ligand 1H8
- In the case of a new and unclassified compound, we need to use grade directly to generate the dictionary, using either the ligands smiles description (preferably) or a mol2 file as input:
grade 'c1(cnc(cc1)C(=O)Nc1cc([C@@]2(C)N=C(OCC2)N)c(cc1)F)C#N' -resname LIG
Grade produces two output files:
- grade-LIG.pdb: idealised coordinates for the ligand.
- grade-LIG.cif: cif format restraint dictionary.
Now that we have prepared the restraint dictionary and have all the required input files, we are ready to run pipedream.
The one remaining check to be made is to ensure that the expected ligand binding site in the reference structure being used does not contain any other compounds. If so, they MUST be removed from the pdb file before use.
In this case, 1w50.pdb is a native structure and the binding site is clear, so no action is required. By default, Pipedream will automatically remove waters from the reference structure, so waters do not have to be removed manually.
Furthermore, there is a single bace monomer in the asymmetric unit, and hence a single binding site.
- Run Pipedream as follows:
pipedream -hklin 4j0p.mtz -xyzin 1w50.pdb -hklref 1w50.mtz -rhofit grade-LIG.cif -postref -d pipe &
- Note that it is not necessary to redirect standard output or indeed standard error to a file. ALL of the output from Pipedream is written into the output directory (pipe in this case).
Now, we will look at the output and the results.
- The primary "standard" output from Pipedream is in summary.out.
- The first section lists information about the job itself - who ran the job, when, where, on what machine and what was the exact command run.
- The second section indicates that the experimental data was an mtz file. It has indicated that it was consistently indexed with the reference mtz file and that the free-R reflections have been copied from the reference data to the experimental data.
- The third section details the outcome of the "limited" molecular replacement step (using Phaser). This step DOES NOT carry out a full unrestricted molecular replacement - only a highly restricted 6 dimensional search to deal with crystal non-isomorphism that is beyond the radius of convergence of rigid body refinement.
- The next section details the outcome of the refinement protocol employed. The default refinement protocol runs two successive BUSTER jobs. The initial R and Rfree and then the R and Rfree after each refinement are listed.
- Following refinement, the outcome of ligand fitting with Rhofit is detailed, giving a summary of the main Rhofit output. All of the solutions that Rhofit finds are listed. In this case, only a single solution has been found.
- Post-refinement results are listed next. Pipedream cannot determine which of the Rhofit solutions are correct and so post-refinement is only carried out on the "best" solution. In this case, there is only one solution!
- Finally, buster-report has been run on the output of the post-refinement and summary.out details where all the output is.
The final R and R free after post-refinement (18.7% and 21.9%) look good. But the proof of the pudding is has the ligand been correctly fit and refined?
So let us look at the final output (and compare it with the deposited 4j0p structure):
visualise-geometry-coot postrefine-grade-LIG
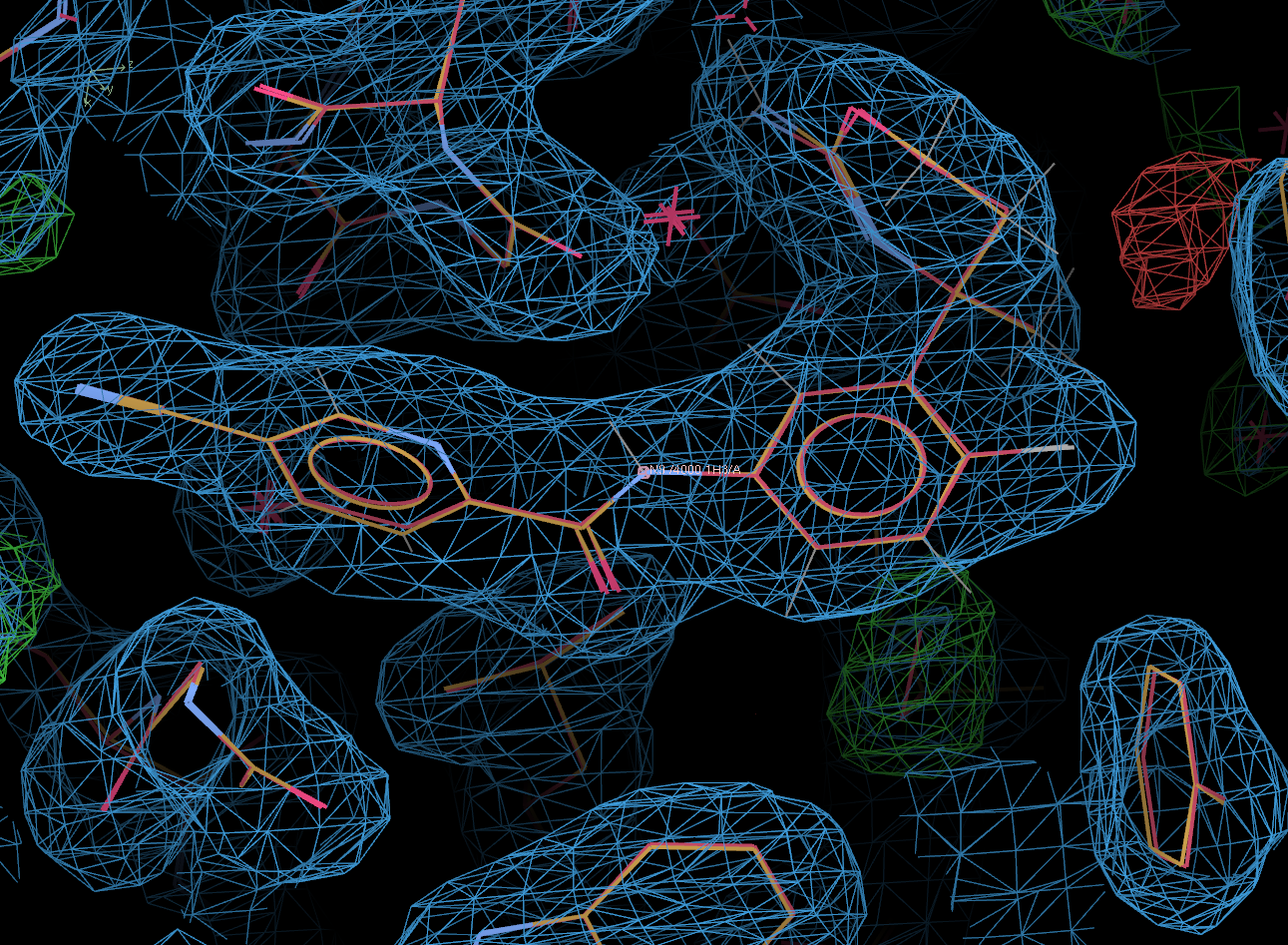
In the above, the original 4j0p model (including ligand) is shown in red.
Clearly, the solution found by Rhofit and post-refined is correct.
- buster-report has been run on the output from post-refinement and we would strongly recommend that you look at the output carefully as it presents a lot of very important and useful information in a clear, pictorial manner and can indicate issues that are not readily apparent (if at all) from more standard refinement statistics. Please see buster-report and ligand analysis for further information on buster-report. The output can be viewed by:
firefox report/index.html
or your favourite browser.
Look at the "Ligand report" tab for detailed analysis of all of the ligands present in the structure, in this case just the ligand fit by Rhofit.
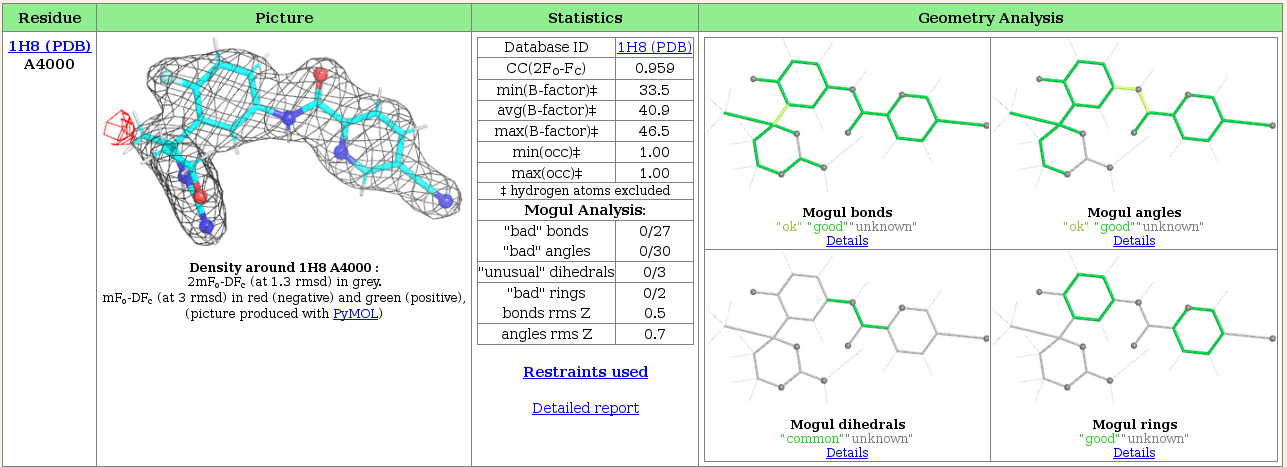
The centre section tabulates information to show how well the refined ligand agrees with the experimental data (the 2fofc correlation coefficient) and standard refinement parameters (Bfactors and occupancy). It also summarizes the Mogul analysis of the refined ligand. A more detailed analysis of the ligand geometry is shown in the "Geometry Analysis" pane on the right hand side, which, using colour coded pictures of the ligand shows Mogul's analysis of bond lengths and angles, dihedrals and rings. The colour coding used is:
- Dark Green - good
- Lime Green - OK
- Pink - poor
- Purple - bad
- Gray - Either the analysis is not applicable (i.e. a feature is not a ring in the Mogul rings pane) or Mogul is unable to render an opinion as there are no similar instances of this feature in the CSD.
In this case, the ligand has very good geometry.
Now look at the "molprobity analysis" tab. This shows the summary table of the Molprobity report for the final structure as well as the Ramachandran plot. A full molprobity chart listing per-residue violations is also available from this page.
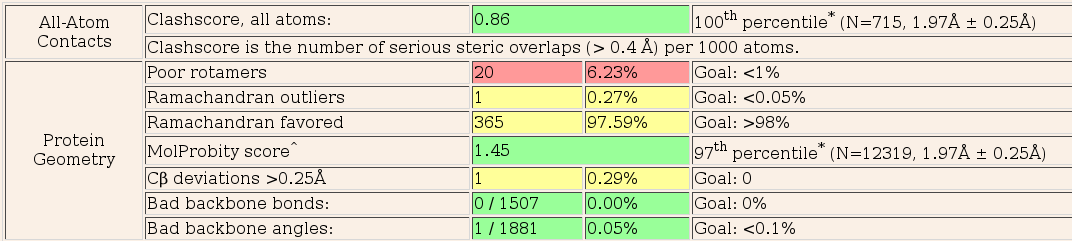
The molprobity analysis is pretty good but clearly there are still improvements that can be made. In particular, there are a number of residues with poor rotamers. These are not necessarily bad but may need looking at. Remember, the structure that is input into Pipedream has come from a different crystal from the experimental data and may also have been treated differently. There may well be conformational differences between the reference and experimental structures (due to a number of causes, not least ligand binding), that cannot be fully rationalised by refinement alone.
IMPORTANT: Pipedream is NOT designed to output a completed, fully refined, deposition ready structure! It may well be necessary to manually correct features of the structure in Coot and then carry out a further re-refinement.