autoBUSTER Example Application: Do -autoncs and TLS reveal an extra peptide in the 1osg structure?
Background
- In a previous example we used 1osg, the structure of the complex between BAFF with the peptide bhpBR3, to show how -autoncs can be useful.
- When looking at the model/maps after after BUSTER refinement with autoncs:
- AutoBusterExample1osgBasicNCS/1osgBasicNCS_003_autoncs_followon_2009_refine.pdb
- AutoBusterExample1osgBasicNCS/1osgBasicNCS_003_autoncs_followon_2009_refine.mtz
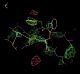
- There is some interesting weak difference density next to peptide K (here contoured at 3.2 sigma):
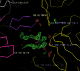
- Note that the density lies between three different asymmetric units (symmetry copies are here shown in yellow). It appears to be about the same shape as the K peptide but is disconnected.
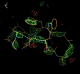
- It was possible to model a peptide into the difference density. Coot was used to manually place the L chain peptide. Side chains were removed and autobuster refinement applied. Difference density peaks were found in the expected positions for 5 side chains in particular the two TRP residues. A refined model of the extra chain can be found here: 1osg_extrapeptide_model_try1.pdb
- This placement was tentative as the density was weak. TLS can be used to improve the difference density making placement easy.
TLS run improves extra peptide density
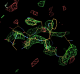
- pdb#: 1osg pdb entry after B-factor refinement with autobuster (atom positions kept fixed).
- So using TLS results in 1.3% drop in Rfree. In addition the Rfree-Rwork gap is slightly narrowed from 4.26% to 4.1%.
- But most importantly the better refinement increases the predictive power of the model to bring out new features. It strengthens difference peaks for radiation damaged disulfide bonds (see tutorial example) but also produces better connected density for the extra peptide (confirming original placement).
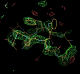
Fo-Fc difference maps
- Compare a refined model for the extra peptide 1osg_extrapeptide_model_try1.pdb with the difference map contoured at 3 sigma. N.B. the extra peptide is not included in any of the refinements.
- it can be noted that this region will have been treated with the bulk solvent correction and so density features will be reduced.
Result pdb and mtz files
Page by Oliver Smart original version 21 July 2009. Address problems, corrections and clarifications buster-develop@globalphasing.com